Client Success Story
AMS Advanced Medical Services strengthens the quality of its clinical studies with BSI CTMS
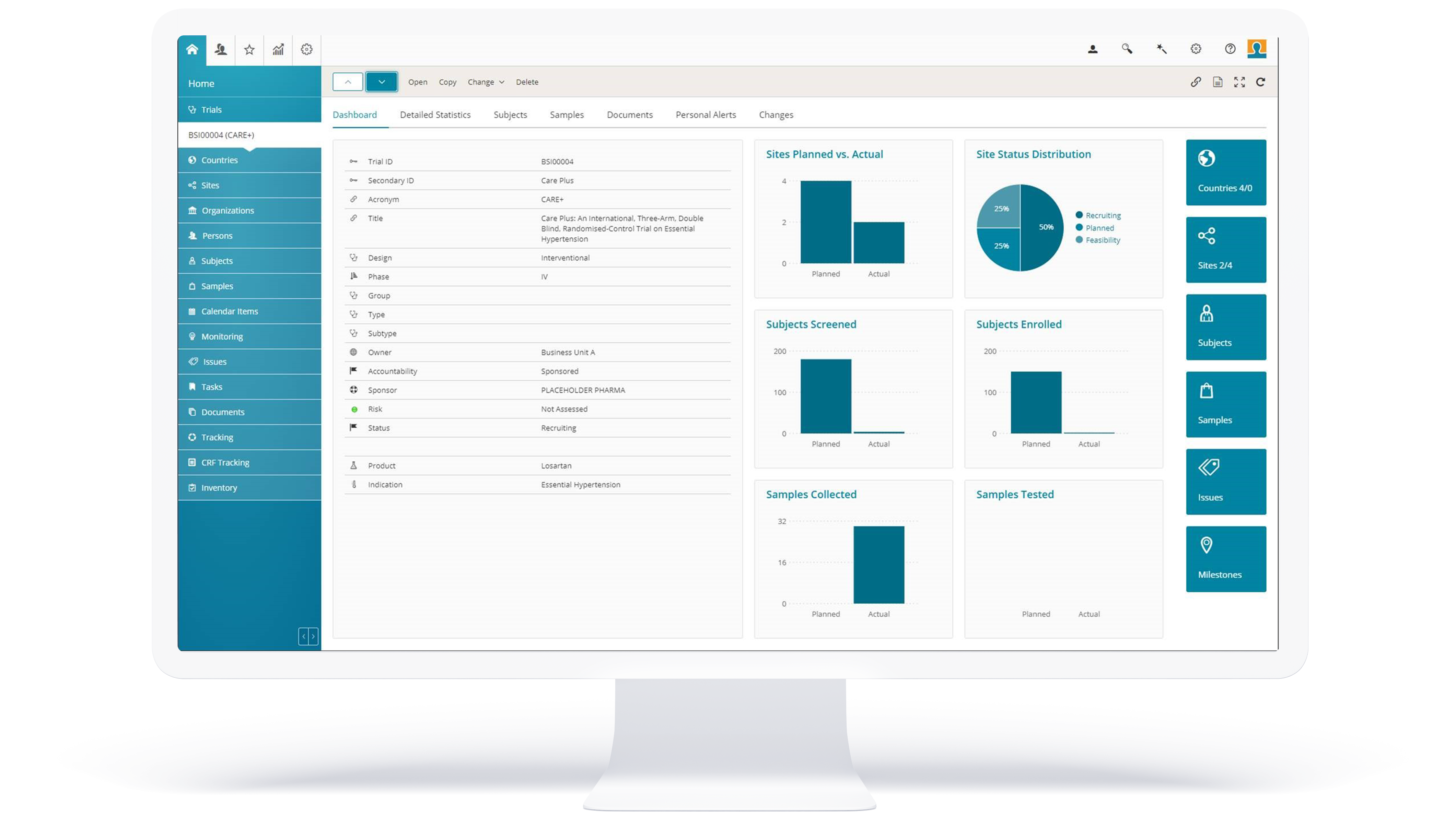
Onboarding a fully-validated CTMS and eTMF in weeks
AMS, based in DE-Mannheim, is a contract research organization (CRO) specializing in the operation of clinical studies in phases I-IV as well as noninterventional studies. For this purpose, AMS always relied on an eTMF (electronic Trial Master File) solution. However, after it no longer showed the usual stability and was in danger of being discontinued, it was time for a change. AMS used this opportunity to upgrade to a complete Clinical Trial Management System (CTMS) from BSI Life Sciences, which, in addition to the integrated eTMF, also has comprehensive monitoring and is customizable to existing processes.

The Challenge
The decision to switch was associated with massive time pressure: The previous software solution was terminated, and a seamless transition had to be implemented within 6 months. In addition, the new solution not only had to be regulatory compliant, but also so user-friendly that all project employees could start immediately without a lengthy training period. The expansion of the level of digitization was also accompanied by a change in corporate culture: away from mountains of paper and filing cabinets towards a paper-free office. Here too, it was important to familiarize the workforce with the new work processes.
The CTMS had to fulfill these criteria
- Fully compliant with both US FDA and EU regulations
- Easy onboarding and use for trial managers an CRAs, both internal and external
- A “one-stop” platform for clinical studies, incorporating an eTMF and eMVR (electronic monitor visit reporting system)
- Highly customizable to existing processes
- A responsive and easily accessible support team
- Smart integration with their current EDC
- Easily validated by the companyʼs QMS (quality management system)
- A solution that provides a rapid ROI

“BSI helped us customize the software so that, for example, we could maintain the same TMF structure that we had used for years. As a result, adapting our standard procedures to the new digital system took very little time and work. In addition, the eClinical system was delivered fully validated.”
Harald Wagner
AMS Director of Clinical Operations
The Results
Extended range of functions and transition to purely digital document processing.
With BSI, parallel operation of an eTMF and a CTMS solution is not necessary; both are integrated into a single software. There is no redundant information and the data only needs to be recorded once, which eliminates sources of error.
A key challenge for which AMS found a solution with BSI CTMS was the configuration and thus use of two different study templates. For a CRO, this is a crucial advantage. This means that AMS is now able to carry out phase I-IV clinical trials as well as noninterventional studies based on individually adapted templates with specific workflows.
In connection with the establishment of the new CTMS solution, AMS also switched from paper documents to electronic processing. “The effect of the paperless office is enormous: we currently have a wall unit freed up almost every month.” Reports Harald Wagner.
The Takeaway
- Switching to new software requires comprehensive planning - especially in clinical research with its longterm planning and investment cycles.
- All the measurable advantages of technical solutions are only of limited help if employees do not accept the solution and are not individually introduced to new methods and processes. This was achieved extremely well when implementing the BSI software.
- In addition to the positive effects such as an expanded range of functions, BSI CTMS also supports AMS in leading and coordinating its employees. The distribution of tasks is simplified; Each employee has a specific role in a study. The system tells the employee which work steps should be completed and by when. It specifies the course of the study and thus creates freedom to concentrate on essential aspects and the specific processing of the study.
- Ease of use also comes into play here and helps to involve all employees - regardless of their experience: a significant advantage in a time characterized by a shortage of skilled workers.
"We are proud to serve well-established and internationally renowned companies like AMS. We know that the adoption of BSI CTMS will make a difference, both for AMS and for its clients, who are mainly clinical trial sponsors in the pharma industry and the academy”
Jan Nielsen
BSI Life Sciences Community Manager

About AMS Advanced Medical Services
Founded in 1997, AMS Advanced Medical Services is an experienced CRO providing a full range of services to clinical trial sponsors, including Project Management, Monitoring, Quality Management, Biostatistics and Data Management, Pharmacovigilance and Medical Writing. AMS has been a European leader in the fields of Market Access, Health Technology Assessments (HTAs) and Rapid Relative Effectiveness Assessments (rapid REAs), and a pioneer in the benefit assessment of medicinal products in Germany. Its customers include world-leading pharmaceuticals, medical devices and biotech companies.
About BSI Life Sciences
Offering smart eClinical software for CTMS, eTMF and more, BSI has been making life science software for real people for 25+ years. Our innovative, user-friendly software solutions are made in Switzerland, Germany, and the United States, and available anywhere a client may require. By focusing resources on delivering functional, compliant, and affordable eClinical software, BSI helps its customers continuously optimize and accelerate the clinical development process, and thatʼs what they love!
