BSI eTMF: The function complete and most affordable eTMF solution for your trial master file management
BSI eTMF (electronic Trial Master File) offers a trend-setting interface and complete trial master file management functionality. It perfectly supports pharma, biotech, MedTech (medical device and in vitro diagnostics) sponsors, as well as CRO and academics (SMO) in their daily work for trial master file management, always being audit and inspection ready. BSI eTMF is being constantly further developed, based on market and regulatory requirements, and in close collaboration with our existing customers. If you are looking for an affordable yet powerful eTMF solution, BSI is the right partner for you.

BSI eTMF Factsheet
Everything you need to know about BSI eTMF on two pages.
Short and sweet, to the point and with convincing arguments.
Companies that count on BSI Life Sciences
Seven good reasons for BSI's electronic Trial Master File
Why is BSI eTMF the right solution for you? Here are seven convincing reasons. If you wish to hear more advantages of our electronic Trial Master File management, we are pleased to organize a demo or a reference call with our customers.
BSI eTMF delivers comprehensive functionalities for all aspects of trial master file management for your clinical trials: from study startup, to execution, flexible reporting and closing. Full support of your own TMF structure or the TMF Reference Model from DIA with controlled access for all study partners e.g. sponsors, CRO and sites. Setup document plans and track the creation, reviewing and quality approval process with enhanced alerting functions. BSI eTMF provides real-time audit and inspection readiness.
With the modern HTML5 web-based user interface, BSI eTMF is one step ahead regarding optics and ergonomics. The feedback that we have received is, that the intuitive interface and the intelligent functions in BSI eTMF make our customers’ daily work easier and more efficient, and that it’s a product that they actually enjoy using. We are confident you’ll find BSI eTMF just as delightful and indispensable.
Due to standardized and proven processes, available pre-configurations, and templates for medical device studies as well as for pharma studies, the onboarding of BSI eTMF for new customers is done in a short time. BSI supports customers after rollout as well as during configuration and rollout.
BSI eTMF is not only attractive when it comes to functionality and usage, but also in operation. The intuitive user guidance keeps training costs minimal, while the easy operation supported by processes accelerates data entry. The open software architecture makes sure that you receive future-oriented innovative software with no dependencies on vendors. Upgrades to new standard versions are included in the SaaS license fees.
With the BSI Portal you can provide access to BSI eTMF for your study sites and investigators for efficient and easy uploading of all site documents relevant for the sponsors trial master file.
Regulatory requirements from 21 CFR Part 11, ICH-GCP E6(R2) and GDPR (General Data Protection Regulation) present major challenges to life science in the areas of patient safety, data protection and security. It is good to know that BSI eTMF is specified and validated according to the regulatory and data protection requirements.
Besides your own access as sponsor or CRO, you can allow controlled access for all study partners, e.g., sites and vendors; and of course, also for inspectors and auditors. This thanks to the sophisticated access right and granting system of BSI eTMF.
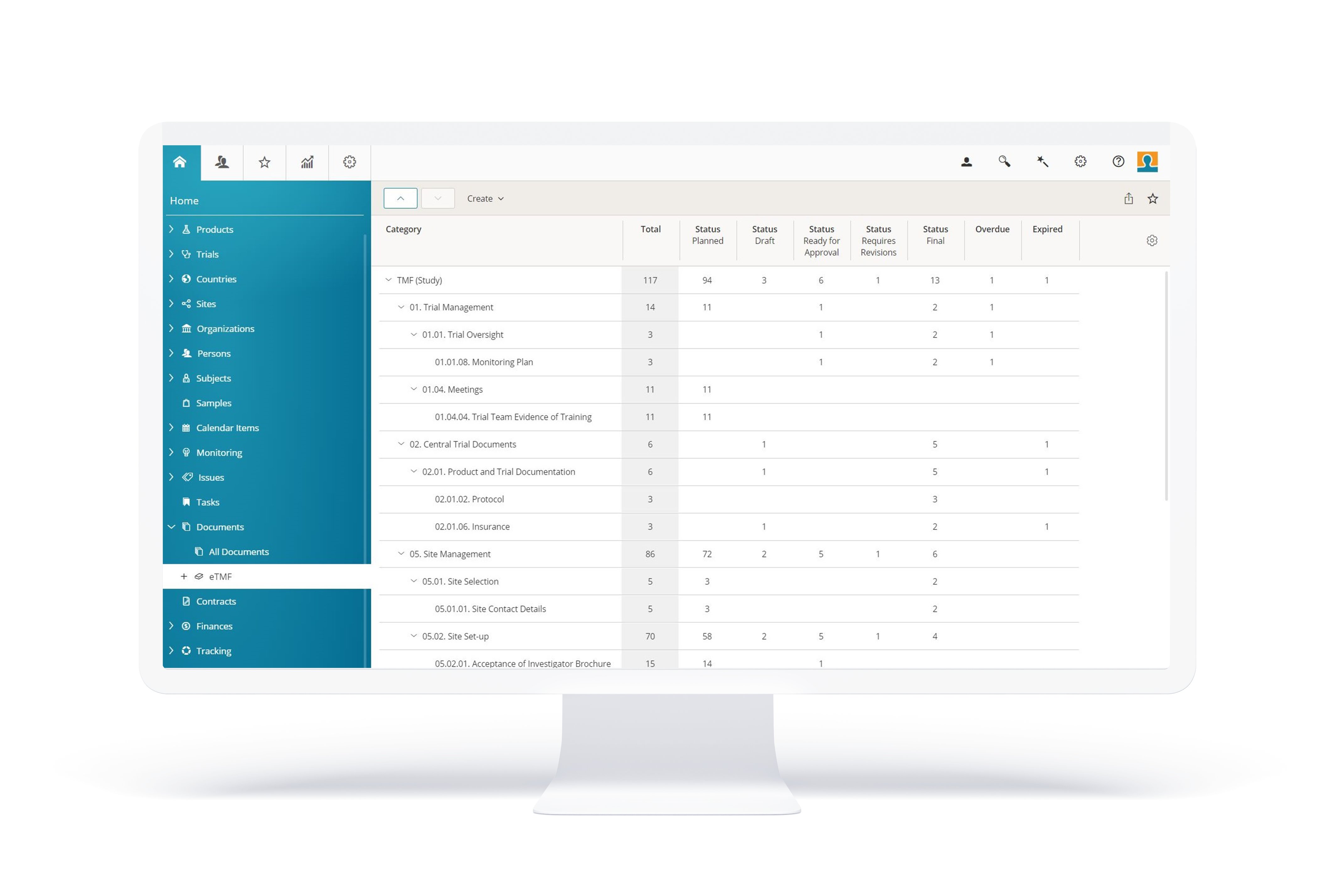
BSI eTMF Functions
BSI eTMF stands out with its comprehensive functionality, providing trial master file management, document plans, reviews and approvals, exports, archiving and a lot more. All this combined with a sophisticated granting and access right system.






















