All-in-one or stand-alone: Our eClinical software solutions cover all aspects of your clinical trials
Whether as Sponsor, CRO or Academic (SMO) - plan and manage all aspects of your clinical trials like patient engagement, clinical trial management (CTMS), trial master file (eTMF), study start-up, and trial supply management (RTSM) with the clinical software solutions from BSI. Do you lack essential components, or would you like to improve the user experience in your existing clinical solutions? We can build and integrate the needed add-ons for you!
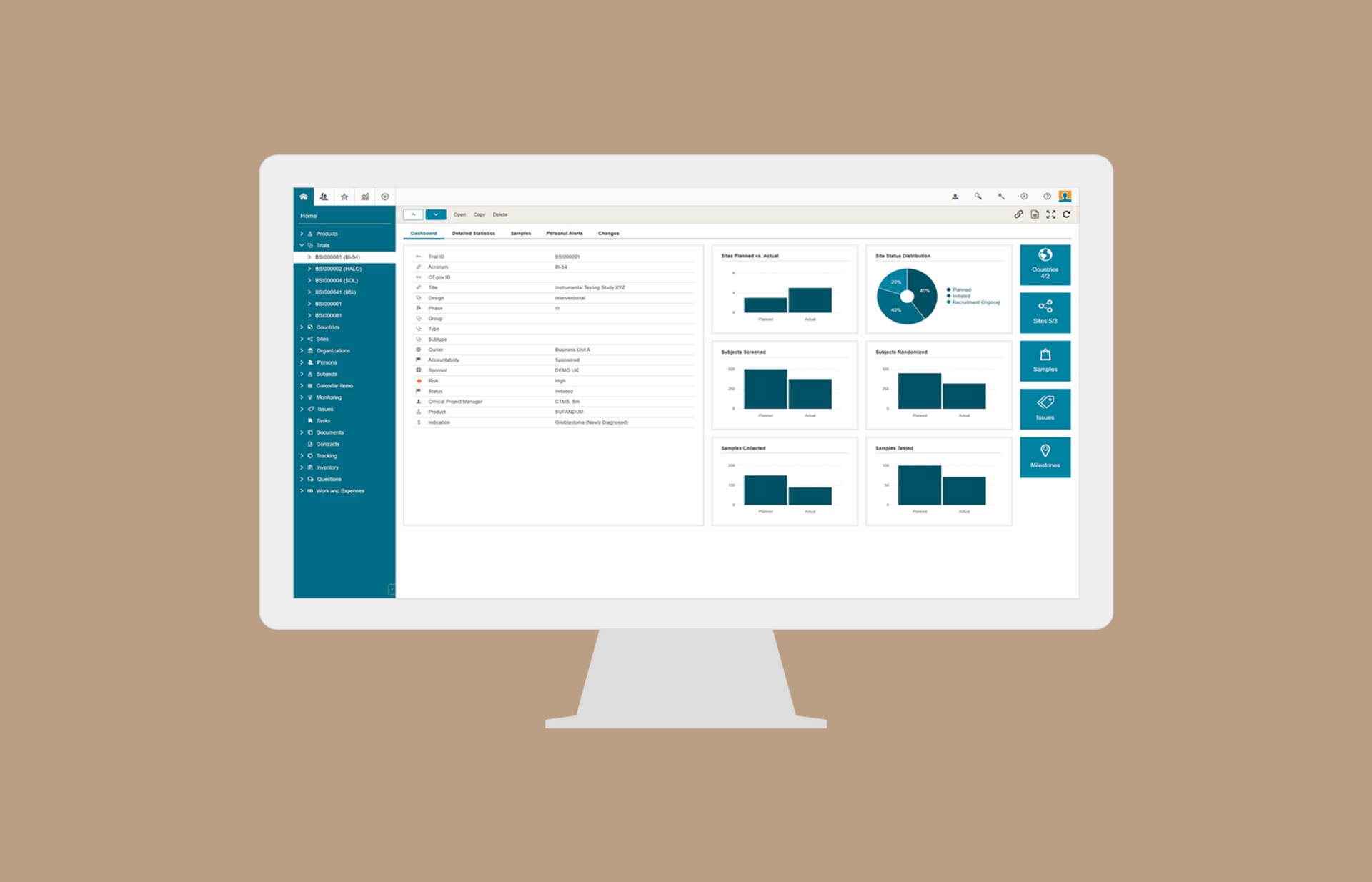
Clinical Trial Management (CTMS)
BSI CTMS is the most innovative, function complete and easy-to-use clinical trial management software on the market. It provides CTMS, eTMF, Study Startup and Trial Supply Management RTSM in one integrated unified platform. Standard interfaces (API) assure complete data oversight and easy integration with external systems (e.g. EDC and eTMF) of your choice. BSI CTMS is the central hub for all aspects of your clinical trials, and is available as software as a service (SaaS) in the BSI Cloud.
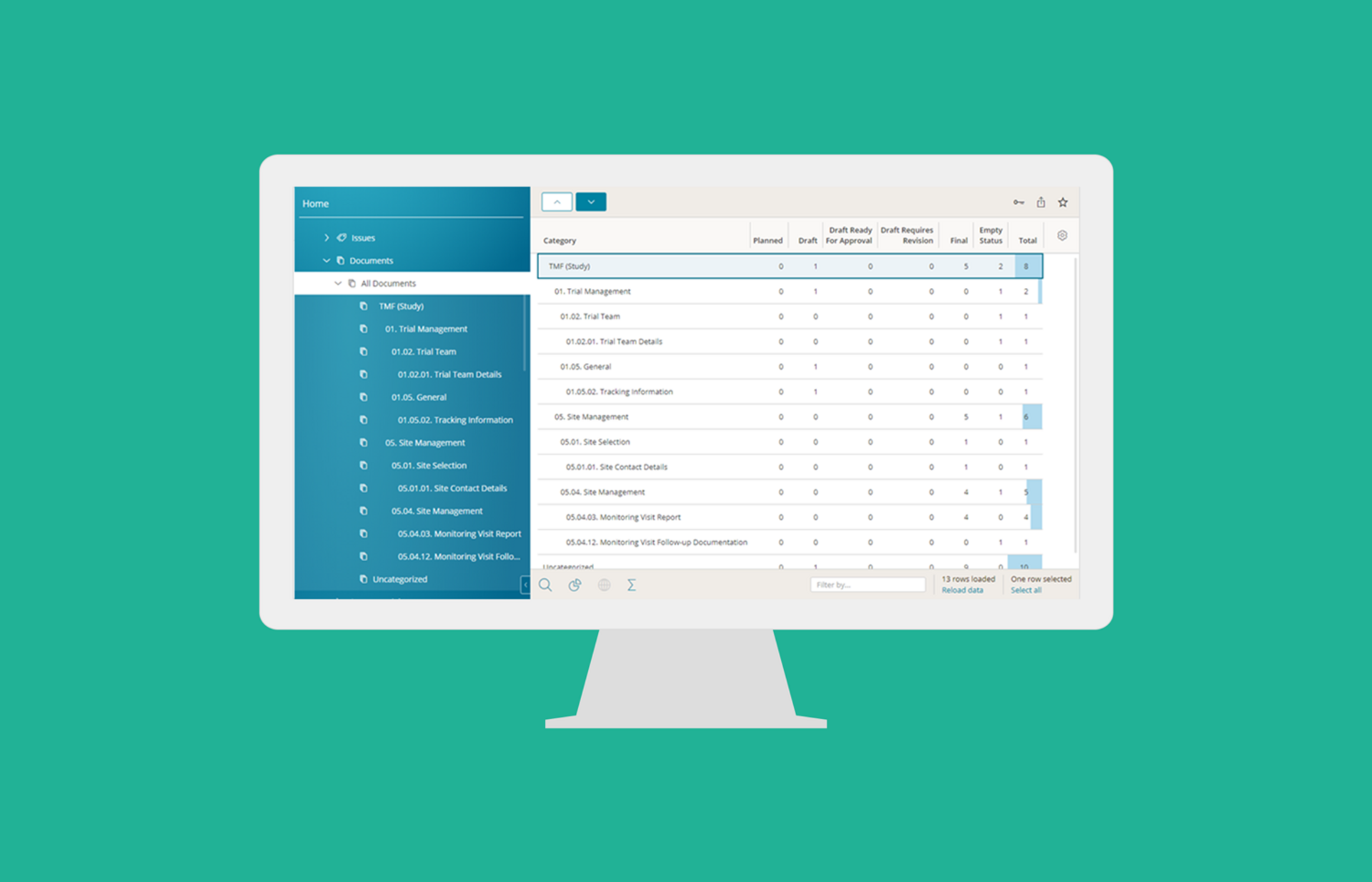
Trial Master File (eTMF)
BSI eTMF is the most affordable and user friendly eTMF solution enabling active electronic trial master file management and real-time inspection readiness. BSI eTMF is provided as stand-alone solution or as integrated part of BSI CTMS. Sponsors, CROs and Sites get online access to efficiently control and manage the eTMF across studies, countries and sites.
Trial Supply Management (TSM)
BSI Trial Supply Management (TSM) provides enhanced randomization and trial supply management functionality and allows you to have your medical and non-medical supply material and accountability under perfect control. The complete inventory of the clinical trials is managed in either global stocks, local warehouses or at site organizations. Did you know? BSI TSM is an integrated part of BSI CTMS.
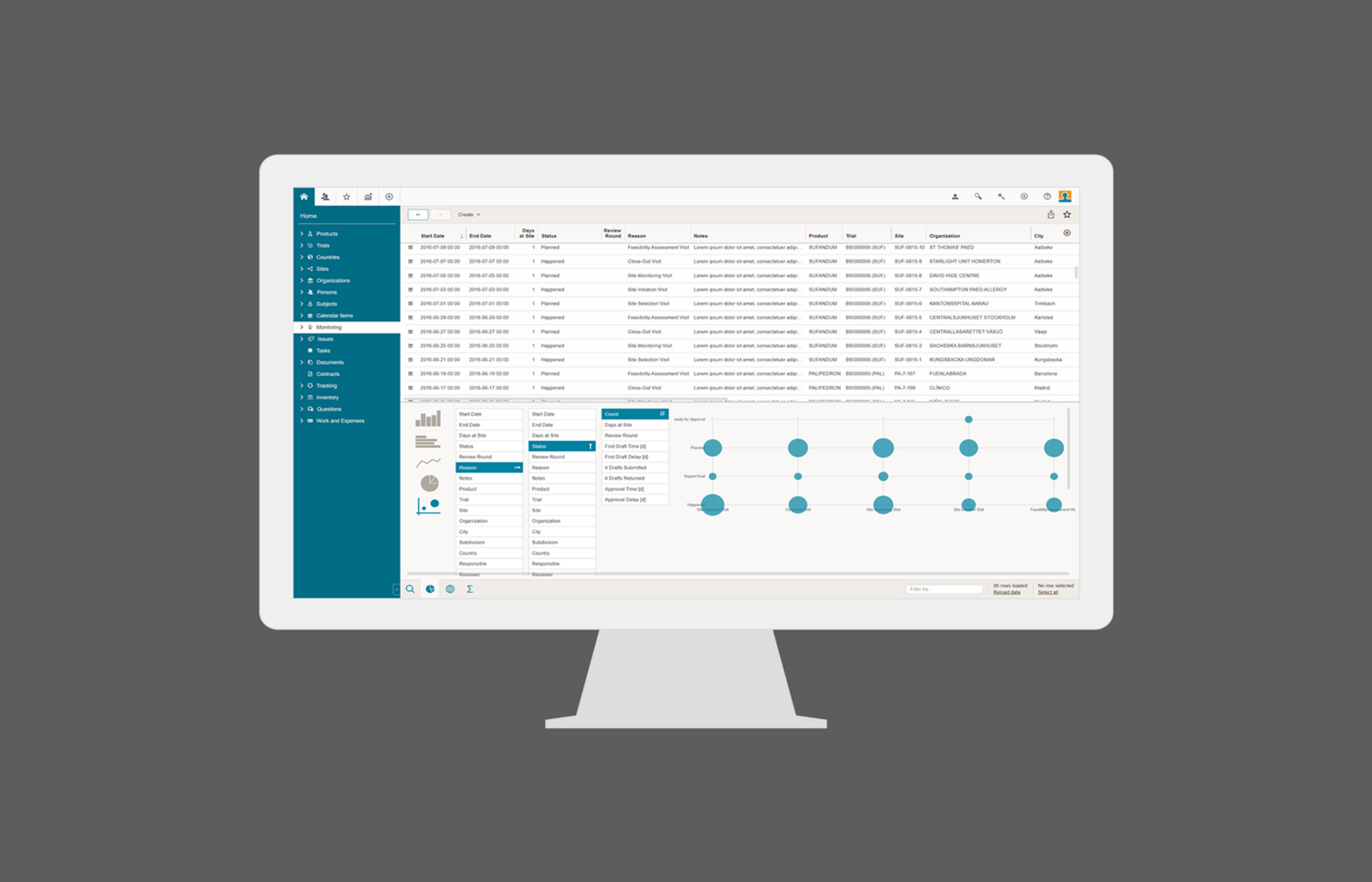
Risk Based Monitoring (RBM)
BSI Risk Based Monitoring (RBM) module enables efficient risk identification, planning and mitigation with cross-functional collaboration among all stakeholders of your clinical trials. The identified risks and the related action items (issues) are used for automatic calculation and optimization of the site monitoring visits. The integrated reporting allows real-time overview of risks and action items from trial level down to site level. By the way: BSI RBM is an optional integrated part of BSI CTMS.
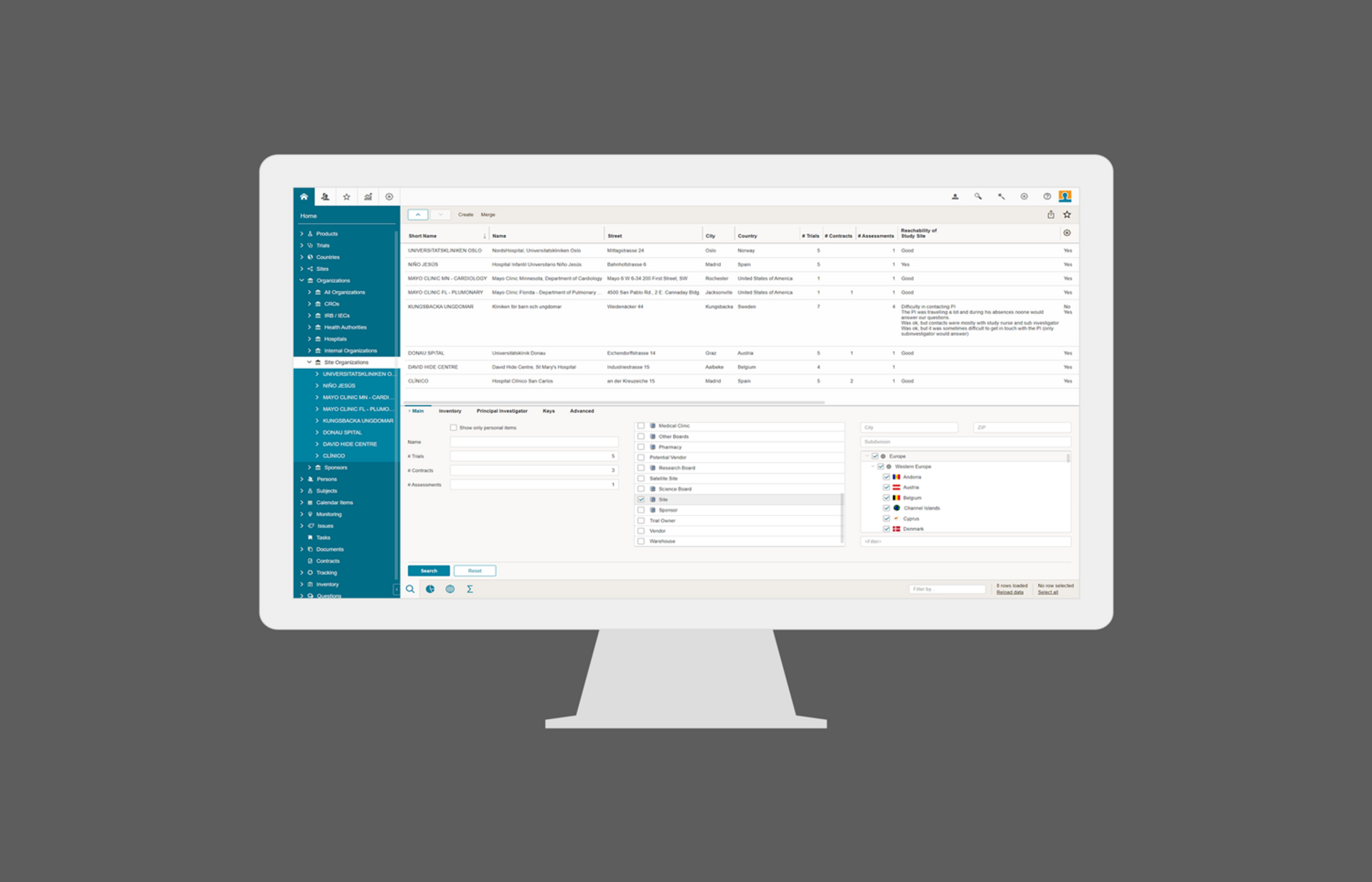
Study Startup
BSI Study Startup’s data driven approach enables efficient study and site startup by allowing planning and sharing of information and documents among all stakeholders. Easy identification, selection and initiation of sites with good performance records and an integrated investigator database. Draw on knowledge captured by site performance assessments and other critical information. Good to know: BSI Study Startup is an integrated part of BSI CTMS and BSI eTMF.

Get a handle on regulatory requirements
Regulatory requirements from 21 CFR Part 11, ICH-GCP E6(R2) and GDPR (General Data Protection Regulation) present major challenges to life science in the areas of patient safety, data protection and security. It is good to know that BSI Life Science software solutions are specified and validated according to the regulatory and data protection requirements.
Additional Add-On’s
You do not like your existing CTMS or other clinical solutions, but cannot get rid of them? We fill in the gaps. With our know-how about life science software for clinical trials, we know precisely what you and your users need. We are happy to supplement your existing solutions with the missing components.
