BSI CTMS: The most modern and efficient tool for your clinical trials
BSI CTMS (Clinical Trial Management System) convinces with his trend-setting interface and the intelligent visualization of data. Behind the optics, our clinical trial management software is packed with functions that support pharma, biotech and diagnostics sponsors, as well as CRO and academics (SMO) in their daily world for managing clinical trials in a targeted way. BSI CTMS is being constantly further developed, based on market requirements and in close collaboration with our existing customers.

BSI CTMS Factsheet
Everything you need to know about BSI CTMS on two pages.
Short and sweet, to the point and with convincing arguments.
Companies that count on BSI Life Sciences
Seven good reasons for BSI's Clinical Trial Management System
Why is BSI CTMS the right solution for you? Here are seven powerful reasons. Our customers also confirm the advantages of our clinical trial management software. We will be pleased to organize a reference call with them.
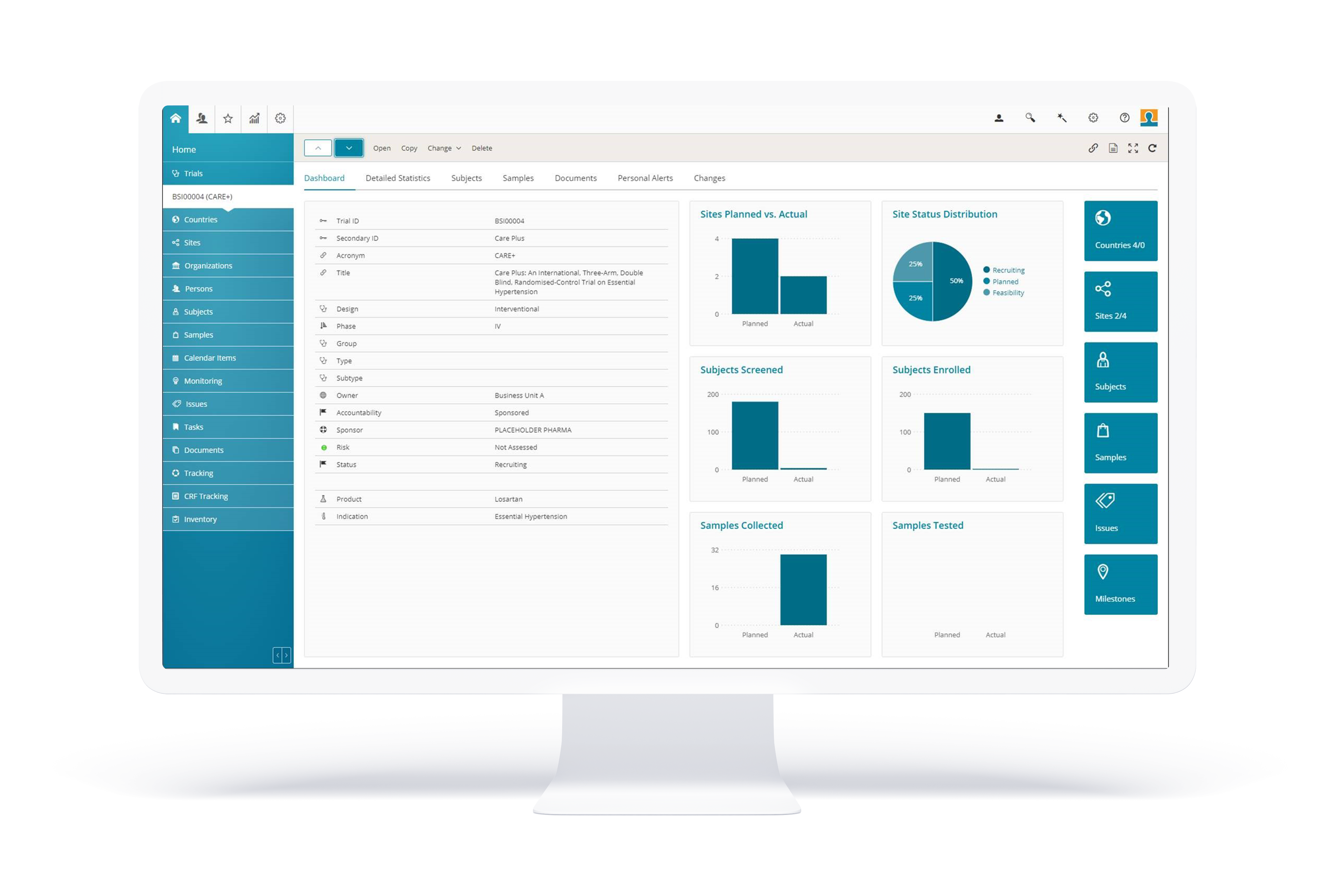
BSI CTMS delivers comprehensive functionality for all aspects of clinical trials: from study startup, to execution, flexible reporting and closing; along with the management of sites and investigators, integrated active trial master file (eTMF) and trial supply management (TSM), suitable for local, regional and global trials. Everything you need is in one software application, configured to the needs of the individual customer.
With the modern HTML5 web-based user interface, BSI CTMS is one step ahead regarding optics and ergonomics. The feedback of our customers proves: The intuitive interface and the intelligent functions in BSI CTMS make our users’ daily work easier and more efficient, and it’s a product that they actually enjoy using. We are confident you’ll find BSI CTMS just as delightful and indispensable.
Due to the BSI standardized and proven processes BSI CTMS can be made available for new customers within very short time. BSI supports the customer during configuration and rollout, no external system integrators are required. As the central “hub” and backbone for your clinical trials management BSI CTMS enables easy integration with other clinical systems. Standard interfaces are available to Veeva eTMF, EDC systems, portfolio and project management tools, clinical data warehouses, active directory for single-sign-on, and more.
BSI CTMS empowers every user with unique flexible reporting functionality. Create your own personal reports using all available data in BSI CTMS (or imported from other integrated systems) by pure configuration. If required, organize and export the data Word, Excel or PowerPoint. Or simply use the out-of-the-box reports.
BSI CTMS empowers every user with our unique flexible reporting functionality. Create your own personal reports using all available data in BSI CTMS by pure configuration and export the data to Word, Excel and PowerPoint. Or simply use the out-of-the box reports.
Regulatory requirements from 21 CFR Part 11, ICH-GCP E6(R2) and GDPR (General Data Protection Regulation) present major challenges to life science in the areas of patient safety, data protection and security. It is good to know that BSI CTMS is specified and validated according to the regulatory and data protection requirements.
Besides your trials, with BSI CTMS you can also plan and manage all other projects and assignments. With the modules for business development, contract and activity management as well as invoicing tracking and human resource management recording, BSI CTMS becomes a real ERP system for contract research organizations (CROs).
BSI CTMS Functions
BSI CTMS stands out with its comprehensive functionality, providing Clinical Trial Management System (CTMS), electronic Trial Master File (eTMF), Study Startup and Trial Supply Management (TSM) in one integrated unified platform. It’s time to say goodbye to Excel and other stand-alone solutions.